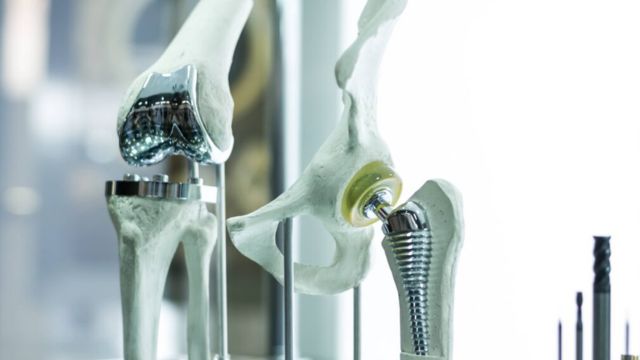
Regarding medical equipment, biocompatibility is a crucial consideration for producers they cannot afford to ignore. Medical equipment either directly or indirectly comes into touch with the human body by nature. This implies that the components utilized ought to not produce side effects or damage in the body. Biocompatibility guarantees that these technologies operate as expected without causing adverse biological reactions, therefore avoiding patient damage and legal problems.
This paper explores the ideas of biocompatibility in medical device creation, the guidelines to be followed, the tests carried out, and the important issues producers have to give top priority.
Understanding Biocompatibility
Simply said, biocompatibility is a material’s capacity to execute with the suitable host response under application. This entails knowing how materials respond in contact with tissues, blood, or another body fluid. In the production of medical devices, this covers both non-implantable (like catheters, syringes, and diagnostic equipment) and implantable (like pacemakers or joint replacements).
A biocompatible device must exhibit:
- Non-toxicity: It should not release harmful substances that can lead to infection, inflammation, or other health risks.
- Non-carcinogenicity: The material should not increase the risk of cancer.
- Non-allergenic: It should not trigger allergic reactions, which can result in skin irritation or more severe symptoms.
- Stability: The material should not degrade over time when in contact with body fluids or tissues.
For manufacturers, ensuring biocompatibility involves using materials and manufacturing processes that adhere to these requirements.
Why Biocompatibility Matters

Biocompatibility is not just a regulatory concern—it’s a vital part of patient safety. If a device or component causes harm, it can result in complications, rejection of the device, or even life-threatening conditions. Beyond patient safety, non-compliant devices can lead to expensive recalls, lawsuits, and damaged reputations.
Key Biocompatibility Testing
The ISO 10993 series of standards is the most widely accepted set of guidelines for testing the biocompatibility of medical devices. The standards cover a wide range of biological testing, including:
- Cytotoxicity Testing
This test evaluates whether a material causes damage to cells. Cytotoxicity is typically tested by exposing cells to the material and observing the cell’s viability and proliferation. - Sensitization Testing
Sensitization tests are designed to evaluate whether the material can cause allergic reactions when it comes into contact with the skin or mucous membranes. - Irritation Testing
This determines whether the material induces any form of irritation, whether acute or chronic, at the site of contact. - Acute Systemic Toxicity Testing
This test determines whether the device or its components can induce toxic reactions when introduced into the body. - Hemocompatibility Testing
For devices that come into contact with blood (e.g., catheters, stents), testing is conducted to ensure the material does not cause clotting, hemolysis, or other blood-related issues. - Implantation Testing
For implantable devices, testing involves placing the material into living tissue to assess how the body reacts to it over a longer period.
Materials Used in Biocompatible Devices
Manufacturers often select materials for medical devices based on their biocompatibility. Some common materials used include:
- Silicone: Known for its flexibility and non-reactivity, silicone is used in many medical devices like catheters and implants.
- Stainless Steel: Stainless steel is biocompatible and is often used in surgical instruments, implants, and orthopedic devices.
- Titanium: Titanium is favored for orthopedic implants, dental devices, and joint replacements due to its excellent biocompatibility and strength.
- Polyethylene (PE): This plastic is often used in medical devices like syringes and prosthetics.
- Polymers (e.g., PEEK): Polymers like PEEK are often used in spinal implants, offering high strength and excellent biocompatibility.
Regulatory Considerations
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) enforce stringent regulations when it comes to biocompatibility. Manufacturers must demonstrate that their devices meet ISO 10993 requirements before gaining approval for marketing.
- The FDA typically requires premarket notification (510(k)) or premarket approval (PMA) for medical devices, where biocompatibility data plays a key role.
- Risk management is also a crucial part of the regulatory process, where manufacturers are required to assess potential risks and mitigate them.
Challenges in Biocompatibility Testing
Biocompatibility testing can be complex due to:
- Material variability: Even slight changes in material composition or processing can affect biocompatibility.
- Device complexity: Devices made from multiple materials require more comprehensive testing to ensure compatibility between them.
- Long-term testing: Long-term effects of a material on the body (for implantable devices) are harder to predict and test, requiring more extensive and sometimes animal-based trials.
Conclusion
Manufacturing medical devices depends on biocompatibility in great part. Manufacturers can guarantee that their products not only satisfy legal criteria but also give patient safety by adhering to correct testing criteria and employing safe components. Medical equipment can be made to interact peacefully with the human body by means of appropriate techniques, therefore producing improved results and less complications.
At J and J Supplies, we offer insightful analysis of the realm of medical supplies and clinical engineering. Keep informed with the newest ideas, news, and advice from the industrial and healthcare sectors. Stay in touch with us for further in-depth stories and professional guidance if you’re searching for the best materials!
FAQs
What is biocompatibility testing?
Biocompatibility testing evaluates whether a material used in a medical device is safe for use with the human body. It checks for potential toxicity, irritation, sensitization, and other adverse reactions.
Why is biocompatibility important in medical devices?
Biocompatibility ensures that medical devices don’t cause harm to the body when they come in contact with tissues, blood, or organs, which is crucial for patient safety and regulatory approval.
What materials are commonly used for biocompatible devices?
Common materials include silicone, stainless steel, titanium, and polymers like PEEK, all of which are known for their non-toxic and non-irritating properties.
How long does biocompatibility testing take?
Biocompatibility testing can range from a few weeks to several months depending on the type of device, the materials used, and the nature of the tests required.
Are there any certifications for biocompatible medical devices?
Yes, ISO 10993 is the primary international standard for biocompatibility testing, and devices must meet these requirements to be compliant with FDA and other regulatory authorities’ standards.