From a conceptual prototype to full-scale manufacture, a medical device travels a complicated, highly regulated path requiring careful planning. Every phase of the medical device development is crucial to guarantee that the end result is consistent with regulations, safe, and efficient. The key stages of this lifetime will be discussed in this paper together with a thorough guidance for manufacturers, engineers, and other interested parties in medical device manufacture.
1. Conceptualization and Design
Every medical device manufacturing process starts with a clinical need or notion. Designers and engineers closely work with medical experts in the conception stage to specify the goal, target users, and functional needs of the device. In this phase:
-
Market Research: Identifying gaps in existing products and user needs.
-
Feasibility Analysis: Evaluating technical viability and regulatory pathways.
-
Preliminary Design: Sketching out initial concepts and functionalities.
The goal is to create a detailed design specification that balances innovation with practicality.
2. Prototype Development
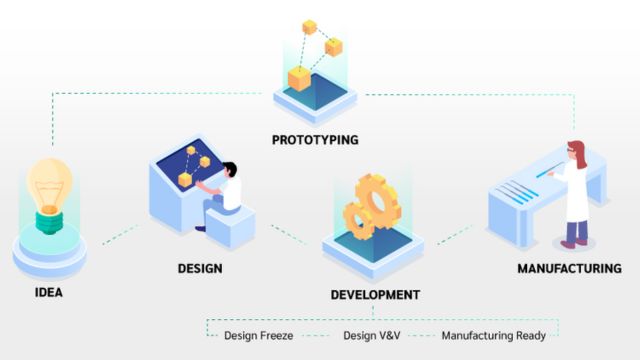
Once the design specifications are finalized, the next step is building a prototype. Prototyping serves multiple purposes:
-
Proof of Concept: Demonstrate that the device can perform its intended functions.
-
Testing and Validation: Identify design flaws or performance issues.
-
User Feedback: Obtain input from clinicians and end-users to refine usability.
Prototypes can be created using various methods such as 3D printing, CNC machining, or manual assembly, depending on the complexity and materials involved. Rapid prototyping accelerates this phase, allowing for iterative improvements.
3. Design Verification and Validation (V&V)
Verification and validation are critical quality assurance steps ensuring the device meets its intended design requirements and performs safely in the intended environment.
-
Design Verification: Confirms that the device design outputs meet the design inputs through rigorous testing, including bench tests, simulations, and inspections.
-
Design Validation: Ensures the device fulfills user needs in real-world conditions, often involving clinical trials or pilot studies.
Documenting all V&V activities is essential for regulatory submissions and audits.
4. Regulatory Approval and Compliance
Medical devices are subject to strict regulatory oversight to protect patient safety. Before scaling production, manufacturers must obtain necessary approvals from authorities like the FDA (U.S.), EMA (Europe), or other regional bodies. This involves:
-
Preparing detailed technical documentation including risk analysis, testing reports, and manufacturing processes.
-
Submitting pre-market notifications (e.g., 510(k) or PMA for FDA).
-
Implementing a Quality Management System (QMS) compliant with standards such as ISO 13485.
Regulatory timelines can vary widely; early engagement with regulators is advisable to streamline approval.
5. Process Development and Scale-Up
Scaling from prototype to production involves developing reliable, repeatable manufacturing processes that maintain quality at higher volumes.
-
Process Design: Define manufacturing workflows, assembly steps, and equipment needs.
-
Process Validation: Demonstrate that the manufacturing process consistently produces devices meeting specifications.
-
Supplier Qualification: Vet and approve suppliers for critical components and raw materials.
Automation and lean manufacturing techniques often come into play here to optimize efficiency and reduce costs.
6. Production and Quality Control
Full-scale production requires strict adherence to quality controls to ensure every unit meets safety and performance standards.
-
In-Process Inspections: Regular checks during assembly to catch defects early.
-
Final Product Testing: Functional and safety tests on finished devices before packaging.
-
Traceability: Implement lot tracking and serial number systems to manage recalls if needed.
Continuous monitoring of production metrics helps in identifying trends and maintaining compliance.
7. Post-Market Surveillance and Continuous Improvement
Manufacturing does not end with product shipment. Post-market activities are crucial for long-term success.
-
Customer Feedback: Collect user experience data to identify potential improvements or issues.
-
Adverse Event Reporting: Monitor and report any device-related problems to regulators.
-
Product Updates: Implement design or process changes based on real-world data.
This ongoing cycle supports product safety, regulatory compliance, and market competitiveness.
Conclusion
From prototype to full-scale production, negotiating the medical device manufacturing lifecycle calls for knowledge, careful planning, and strict quality control. Manufacturers may effectively launch creative and safe medical devices to market by using a disciplined strategy covering design, prototyping, regulatory compliance, and production scaling. Success in this very specialized area depends on constant cooperation among engineers, doctors, regulatory experts, and quality teams.
Knowing each stage of the lifecycle will enable you to foresee difficulties and properly streamline your production line whether you work in medical device manufacture or intend to enter this field.
Looking for reliable medical, clinical engineering, or manufacturing supplies? At J&J Supplies, we provide high-quality products tailored to your industry needs. Explore our extensive range and experience exceptional service and support. Contact us today to find the perfect solutions for your business!